Integrating wearable accelerometer data with machine learning for enhanced osteoarthritis diagnosis – a feasibility study
Bone and joint diseases such as osteoarthritis, a condition where joints become painful and stiff, are common problems. Osteoarthritis alone impacts over 500 million people around the world and 10 million in the UK, leading to high medical costs. Accurate diagnosis and understanding of these conditions are important to develop more treatments.
In this study, we will use wearable devices to measure movement whilst walking and to measure everyday activity patterns. By combining this movement information with other clinical information and using advanced computer programs (machine learning) to analyse the data, we aim to identify and monitor osteoarthritis more accurately.
For this study we will ask 60 people to take part. The aim is to see how effectively these measurements help us detect osteoarthritis in the knee or hip. This study will guide future research with a larger group of people. In the long term, this research could improve diagnosis, treatment, and patients’ quality of life.
Chief Investigator
Professor Andrew McCaskie – awm41@cam.ac.uk
Principal Investigators
Dr Kirsten Rennie – kirsten.rennie@mrc-epid.cam.ac.uk
Mr Simone Castagno – sc2257@cam.ac.uk
Study Coordinator
Mrs April Arnold – april.arnold@mrc-epid.cam.ac.uk
Adults aged 18 or older who are diagnosed with knee or hip osteoarthritis AND healthy individuals for comparison. Participants will be recruited from a single centre study at Cambridge University Hospitals NHS Foundation Trust.
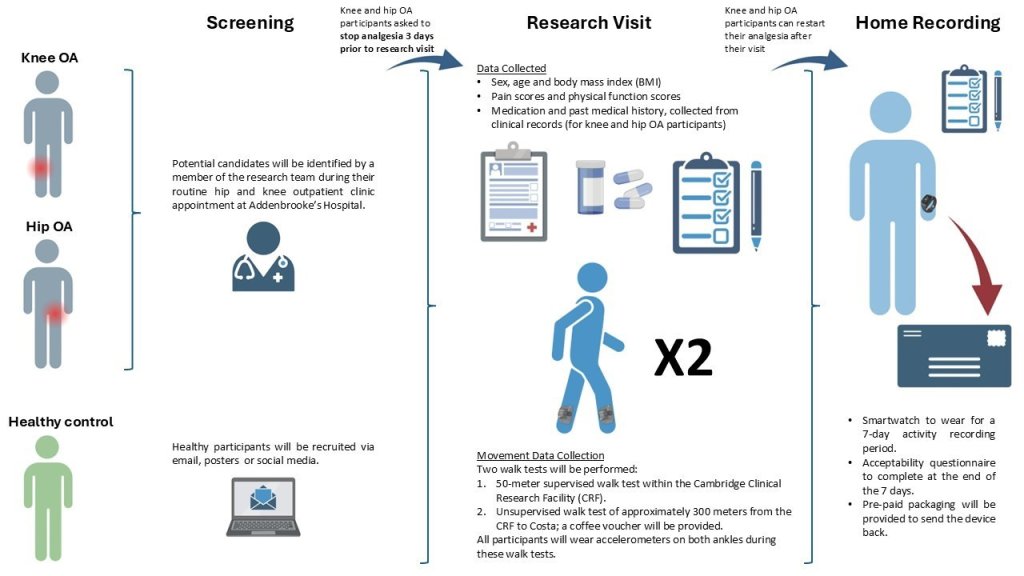
Recruitment
Potential candidates are screened during their routine hip and knee outpatient clinic appointment at Addenbrooke's Hospital, and healthy participants are recruited via email, posters and social media.
Knee and hip OA participants are asked to stop analgesia 3 days prior to research visit.
Data collection
In the research visit the data collected includes:
Sex, age and body mass inded (BMI)
Pain scores and physical function scores
Medication and past medical history, collected from clinical records (for knee and hip OA participants).
Movement Data collection - two walk tests will be performed:
50-meter supervised walk test within the NIHR Cambridge Clinical Research Facility (CRF)
Unsupervised walk test of approximately 300 meters from the CRF to Costa; a coffee voucher will be provided. All participants will wear accelerometers on both ankles during these walk tests
Knee and Hip OA participants can restart their analgesia after their visit
Home recording includes:
A smartwatch to wear for 7-day activity recording period
Acceptibility questionnaire to complete at the end of the 7 days
Pre-paid packaging will be provided to send the device back
If you would like more information on this study please get in touch at: AMALGAM.study@mrc-epid.cam.ac.uk

